The fastest-developed vaccine in history
As the virus raced around the world, the need for mass immunization became glaringly clear.
Yet this urgent goal was not without its challenges. New vaccine development takes an average of 10 years. How could that process be sped up while keeping the scientific rigour to produce safe vaccines? How could it be possible to protect the world from a virus that seemed to be spreading at breakneck speed?

A process that takes an AVERAGE OF 10 years and has never taken less than 4 years was compressed to 11 months
Standard vaccine development involves a series of clinical trials that usually take years to be carried out in sequence.
However, with COVID-19 spreading like wildfire, financial and scientific resources were poured into the development of hundreds of vaccine candidates, and the stages of clinical trials were overlapped rather than run in sequence to expedite the process, while keeping at the forefront the safety assessment of the vaccines.
Comparing the pace of COVID-19 vaccine development to traditional vaccine development
The Vaccine Development Process

1. Funding & resource allocation
Beginning in 2020, billions of dollars from national governments, global organizations, NGOs and philanthropists are channeled into research and development.
2. initial Development
A vaccine is designed and developed and put through preclinical testing. The main focus is on making vaccines that are safe and effective and can be manufactured at scale.

3. Clinical trials & quality control measures
The vaccine enters clinical trials where they are tested in groups of people to assess safety, immune response,and efficacy.

4. Authorization
Just 326 days after the virus's genomic sequence was published, WHO issued the first Emergency Use Listing Procedure (EUL) authorization for the Pfizer-BioNTech vaccine. Additional vaccines were approved in the following weeks and months, and the global vaccination campaign was under way.

5. Manufacturing
Following approval, pharmaceutical companies progress swiftly into safe mass production of the vaccine. When ready, the vaccine is bottled in glass vials and carefully packaged for safe, temperature-regulated storage and transport. In order to meet the sudden demand for COVID-19 vaccine manufacturing, there was an unprecedented expansion of production networks and global industry collaboration.
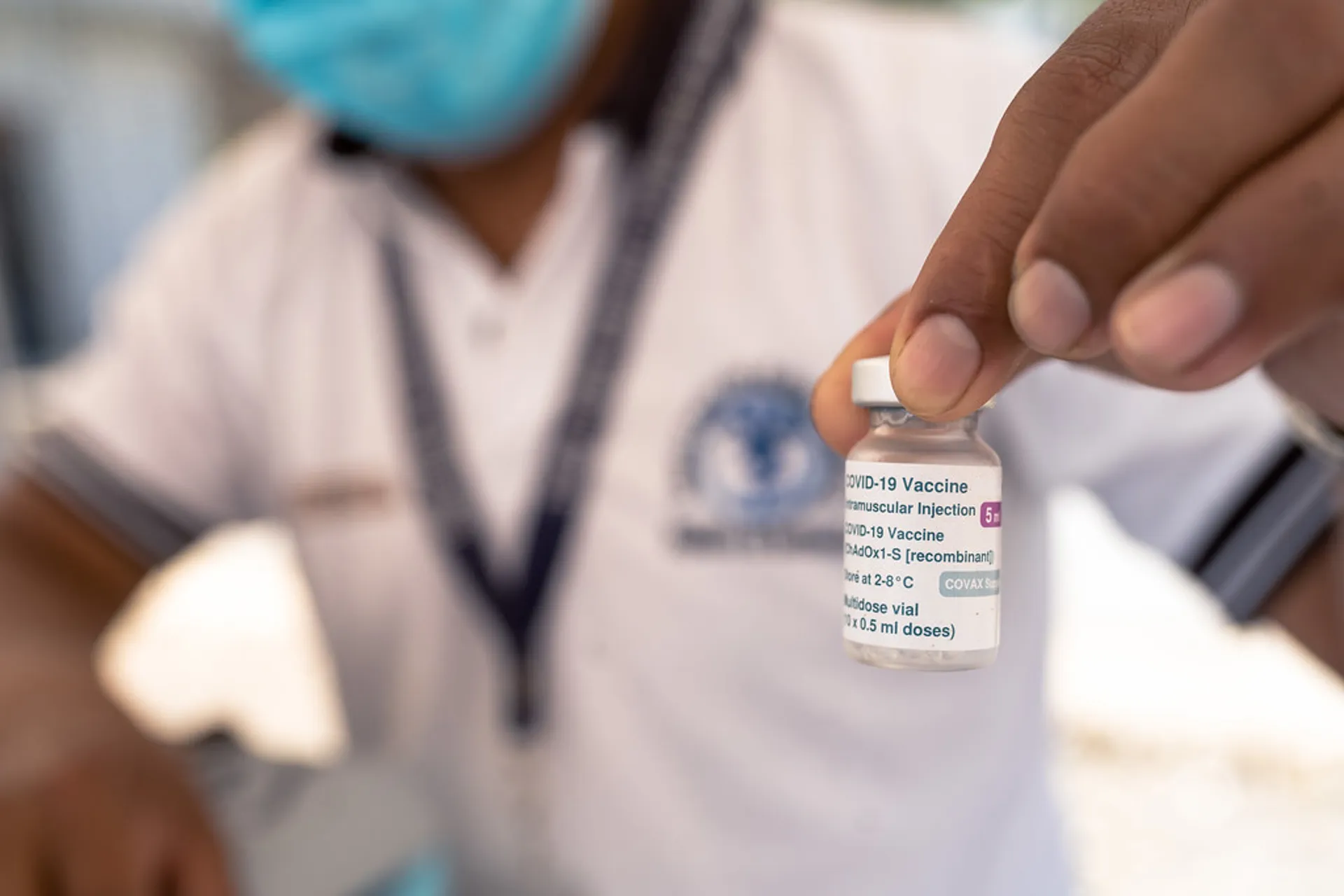
6. Licensing
National Regulatory Authorities had to authorize vaccines based on clinical trial data on safety and efficacy and on quality of manufacturing. As with every new vaccine, countries prioritized the establishment of vaccine safety surveillance systems to monitor potential adverse events.

7. Supply & Distribution
Once a purchase agreement is in place, manufacturers make vaccines available. The vaccines are then flown to countries in temperature-controlled equipment, where they are stored in cold warehouses and further distributed in vaccine carrier boxes or smaller vaccine carriers to cities, towns and communities for health and care workers to administer.
The cold chain
Those working on vaccine delivery knew from the start that the cold chain — the logistical link storing vaccines safely within a specified low-temperature range over time and distances, often in warm climates and rugged conditions – would be essential to deliver potent vaccines to all corners of the globe.
A humble plastic box – a delivery mechanism that changed the course of history.
The logistical issues involved in keeping glass vials cold for long journeys are as old as vaccines themselves. And even before the pandemic, researchers had been working on developing these technologies further, using new methods like automated drone deliveries or camel-carried passive coolers. One key factor was common to all of these innovations: strengthening countries’ supply and cold chain logistics systems.

MÉNAKA
eastern mali
Distance travelled
1500 km via plane from Bamako
Highest average temperature:
41°C
Vaccine temperature:
+2°C TO +8°C
Innovating at speed
The overwhelming pressure the pandemic put on public health systems demanded an innovative and collective effort.
Once a shipment of vaccines arrives at an airport somewhere in the world, a whole chain of logistics is set in place to get them where they need to go. People on the ground need to be trained. The vaccines need to be kept cold. They need to be transported on vehicles or motorcycles or boats, or on animals. Each and every place in the world had its own unique set of challenges.
Almost Every part of this journey requires meticulous planning
For example, in the United Arab Emirates, a COVAX initiative chartered flights to 21 countries.
Workers drained gas from the ultra-cold-chain freezers in order to safely transport them on planes, only to have to re-gas them at their destinations – costly, but the fastest way to get freezers where they were needed.
On 7 October 2021, the first shipment of 65 ultra-cold freezers touched down for onward distribution to countries including Burkina Faso, Sudan and Zambia. This became a poignant moment in time highlighting what could truly be possible despite the odds.

7 October 2021
First flight facilitated by the Hope Consortium touches down in Belgium.
Journey of a vaccine
Track the first international delivery of COVID-19 vaccines from COVAX to Ghana.
As the pandemic continued, CEPI, Gavi, UNICEF, WHO and other partners worked relentlessly alongside countries to develop safe and reliable paths to keep vaccines moving.
These systems that were set up during the most chaotic and relentless phases of the pandemic will now go on to be the backbone of any public health response that requires vaccination to come. The lesson? How we come together in a crisis defines how we survive in the future.